Overcoming a significant roadblock to predictable ablation of soft tissue, Covidien plc (NYSE:COV) has unveiled an advanced ablation system that offers physicians predictable results regardless of the target location or tissue type. The Emprint™ Ablation System with Thermosphere™ Technology is designed to precisely heat and destroy diseased soft tissue (including liver, lung and kidney), and non-resectable liver tumors.
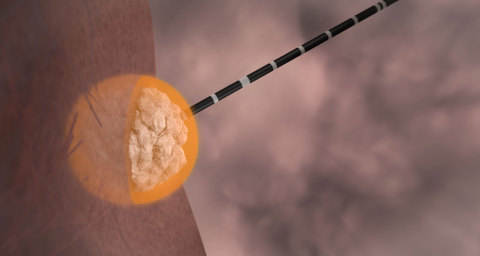
Liver tumor ablation with Emprint(TM) Ablation System can be performed without invasive surgery by inserting the system's antenna into the tumor directly through the skin. The physician utilizes ultrasound or CT scanning to guide the antenna. (Photo: Business Wire)
“Covidien is dedicated to improving patient safety and simplifying procedural options, and this innovative technology enables physicians to deliver precise ablation directly to soft tissue, including liver tumors,” said Chuck Brynelsen, president, Early Technologies, Covidien. “By providing predictable spherical ablation zones, this technology gives physicians more choices in terms of approach, further simplifying needle placement and saving planning and procedure time.”
Surgical removal of tumors may not be an optimal solution for some patients. A liver tumor ablation procedure, which destroys liver tumors without removing them, can be an alternative to open surgery resection or other treatments.
The Emprint Ablation System provides clinicians three kinds of spatial energy control – thermal, field and wavelength – to create predictable and spherical ablation zones regardless of target location, tissue type, or changes in tissue properties during a procedure.
Covidien’s advanced ablation system can be used in three different procedure settings including non-surgical procedures directly through the skin, minimally invasive surgery and open surgery.
Covidien received U.S. Food and Drug Administration 510(k) clearance for Emprint Ablation System with Thermosphere Technology in April 2014. In addition, Covidien completed all European requirements to CE Mark the product. The company expects to fully launch the Emprint system in the United States and the European Union during the current quarter. Please visit http://surgicalsolutions.covidien.com/introducing_emprint to learn more about the Emprint Ablation System.
ABOUT COVIDIEN
Covidien is a leading global health care products company that creates innovative medical solutions for better patient outcomes and delivers value through clinical leadership and excellence. Covidien develops, manufactures and sells a diverse range of industry-leading medical device and supply products. With 2013 revenue of $10.2 billion, Covidien has more than 38,000 employees worldwide in more than 70 countries, and its products are sold in over 150 countries. Please visit www.covidien.com to learn more about our business.
Photos/Multimedia Gallery Available: http://www.businesswire.com/multimedia/home/20140716005186/en/

